Teligent Pharma has introduced a brand new don’t forget that influences its Lidocaine HCl Topical Solution USP 4% anesthetic liquid offered in 50ml glass bottles. The reason, the organization explains, is the ability for “high-quality efficiency,” that means the bottles can also additionally include extra lidocaine than indexed at the label. This, in turn, places customers prone to toxicity.
Lidocaine is an anesthetic regularly offered as the primary aspect in numbing merchandise, inclusive of sprays used to assist lessen sunburn ache, lotions used to lessen burn ache, and gels meant to lessen enamel ache. Though those merchandise, inclusive of comparable ones that include benzocaine, are to be had over the counter withinside the US, they commonly include best very low ranges of the anesthetic because of toxicity risks (thru FDA).
More robust anesthetic merchandise can be made to be had to positive sufferers thru prescriptions, however, for such things as numbing a nasal passage earlier than placing a feeding tube (thru NCBI). One such prescription-best numbing product is Teligent Pharma’s Lidocaine HCl topical solution.
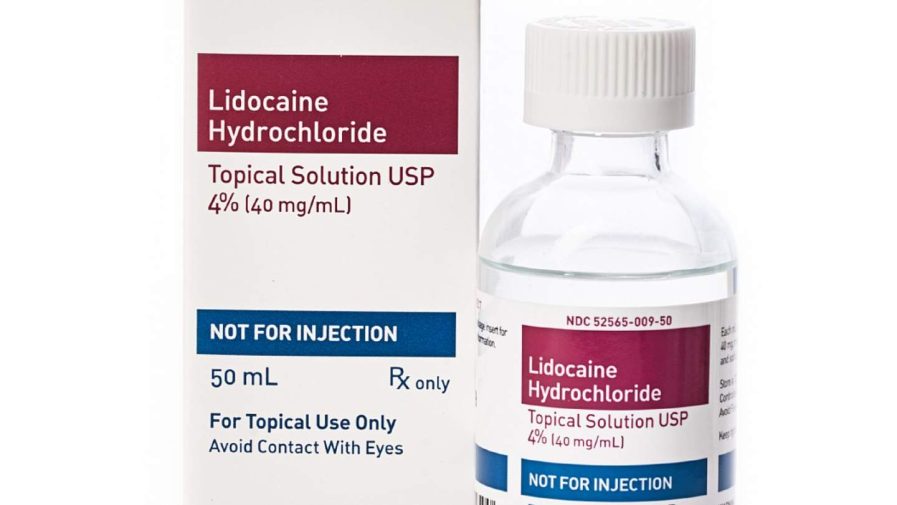
The liquid is offered in a tumbler bottle with a screw cap and — primarily based totally on testing — can be too robust. The high-quality efficiency become detected on the nine-month and 18-month balance timepoints, consistent with Teligent, which says its product is meant to be used as topical anesthesia withinside the “oral and nasal cavities and proximal quantities of the digestive tract.”
Too tons lidocaine and toxicity
Teligent Pharma explains that the use of a product that includes an excessive amount of lidocaine can also additionally bring about a better than meant dose. As a result, it’s far feasible the affected person should increase a circumstance referred to as neighborhood anesthetic systemic toxicity (thru NIH), which can also additionally effect the primary anxious device and cardiovascular systems. Failure to understand and deal with the toxicity should, in a few cases, result in death.
In mild of those risks, Teligent Pharma has recalled the impacted lidocaine bottles and is arranging to have them back from distributors. Any sufferers or purchasers who can be in ownership of a recalled bottle are cautioned to prevent the use of it at once and rather go back it to the shop from which it become obtained for a refund.
Should you be worried?
It’s essential to observe that even as ache alleviation merchandise are offered over the counter, inclusive of a few numbing answers for problems like enamel ache, the bottles blanketed via way of means of this don’t forget had been best to be had thru prescription. If to procure a bottle of liquid anesthetic or different over the counter numbing product, it’s in all likelihood now no longer the identical one recalled via way of means of Teligent.
To make sure, take a look at the product you very own for the brand, manufacturer, and different figuring out details. Two lot numbers are blanketed via way of means of Teligent’s don’t forget, one with an expiration date of 05/2023 and every other of 01/2024. The don’t forget word has been posted at the FDA’s website, inclusive of a image of the product’s label (above), to assist purchasers decide whether or not they’ve one of the recalled bottles.
If you do very own one of the recalled bottles, it’s far very essential to test the don’t forget advisory, observe the don’t forget instructions, and get in touch with the organization and/or one’s healthcare issuer for extra information.